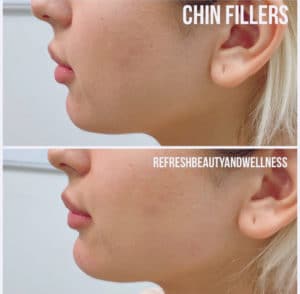
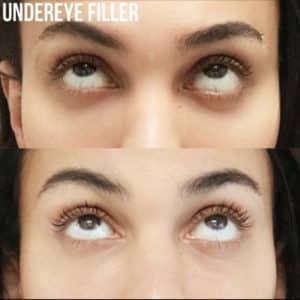
Cosmetic Injectables
Eliminate contraction-induced wrinkles, smooth facial wrinkles, treat etched in lines and wrinkles, plump lips or dissolve fat.

Description: After a quick, gentle set of injections, our aesthetic experts can help you achieve the look you want. Using our vast collection of beauty fillers, we can help you: smooth out facial lines, prevent wrinkles, add volume to your lips, as well as lift and contour the cheeks, under eye area, jawline, and nose. Book a consultation with us today so we can help you look and feel Refreshed!
- Juvederm Volbella
- Juvederm Ultra/ Ultra Plus
- Juvederm Vollure
- Restylane -L
- Restylane Refyne/ Defyne
- Restylane Kysse
- Revanesse Versa
Procedure: In order to make sure our patients are as comfortable as possible during the injection process, a topical numbing cream is applied on the areas of interest. After letting the numbing cream sit for thirty minutes, the injections are then gently given.
Possible Side Effects: Can include slight bruising, swelling, redness, tenderness, and itching. In order to minimize these effects, we give our patients an ice pack to use immediately after the fillers are injected.
Description: This popular injectable needs no introduction as it is used worldwide to soften wrinkles in addition to preventing deep wrinkles from setting. It is most popularly used on areas such as the forehead, crow’s feet, and between the eyebrows. Taking into account how safe and reliable Botox is, it’s easy to see why this injectable is one of our most popular and in demand services!
Procedure: Because Botox is injected through a very small needle, pain is minimal. Calculated units of Botox are quickly and gently given to the targeted areas. Topical anesthetic may be used depending on the patient’s tolerance to pain.
Possible Side Effects: Can include slight bruising, swelling, redness, tenderness, and itching. In order to minimize these effects, we give our patients an ice pack to use immediately after the fillers are injected. Other side effects include headaches, difficulty swallowing, and muscle stiffness.
Used in conjunction with PRP, plasma gel filler is a more natural alternative to beauty fillers as it uses the growth factors straight from the patient’s blood. Like other fillers, plasma gel filler can be used to treat wrinkles, folds, dark spots, acne scars, or increase volume in the face.
Procedure: (See Procedure for Platelet-Rich Plasma). After the platelet-rich plasma is collected, it is concentrated into a gel filler that is gently injected into the selected area.
Possible Side Effects: Can include swelling, bruising, bleeding, minor or no improvement, allergic reactions, scar formation, infection, migration/deformity
Frequently Asked Questions
JUVÉDERM®
Over time, skin loses its volume. JUVÉDERM® helps you temporarily bring it back. Hyaluronic Acid, or HA, is a natural substance in the body that delivers volume to the skin. Modified HA is the main ingredient behind JUVÉDERM® . Getting treated with JUVÉDERM® helps add volume to different areas of the face without surgery while also temporarily restoring volume loss beneath the surface.
Approved Uses:
JUVÉDERM® VOLUMA™ XC injectable gel is for deep injection in the cheek area to correct age-related volume loss and for augmentation of the chin region to improve the chin profile in adults over 21.
JUVÉDERM® VOLLURE™ XC and JUVÉDERM® XC injectable gels are for injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. JUVÉDERM® VOLLURE™ XC injectable gel is for adults over 21.
JUVÉDERM® VOLBELLA™ XC injectable gel is for injection into the lips for lip augmentation and for correction of perioral lines in adults over 21.
JUVÉDERM® Ultra XC injectable gel is for injection into the lips and perioral area for lip augmentation in adults over 21.
The filler is injected with a very small needle directly into the area to be treated. You may experience some stinging or burning as the filler is inserted. A series of treatment sessions may be necessary to fill and smooth lines and wrinkles, or to raise a depressed scar to the level of surrounding skin.
- Most side effects are mild or moderate in nature, and their duration is short lasting (7 days or less).
- The most common side effects include, but are not limited to, temporary injection site responses such as: redness, pain/tenderness, firmness, swelling, lumps/bumps, bruising, itching, and discoloration.
- As with all skin injection procedures, there is a risk of infection. One of the risks with using filler is unintentional injection into a blood vessel. The chances of this happening are very small.
The effects of fillers can last anywhere between three months to two years. It depends on the product being used and where on the body the product is being injected as well as how the body metabolizes the filler.
Restylane®
The Restylane family of products is a brand of hyaluronic acid dermal fillers, which includes Restylane Silk, Restylane Lyft, Restylane, Restylane Refyne and Restylane Defyne. Each is a clear gel formulation of hyaluronic acid specifically formulated to act like your body’s own naturally produced hyaluronic acid. Each Restylane product is designed for a specific purpose.1
Restylane Silk is the first FDA-approved product specifically designed for subtle lip augmentation and the smoothing of wrinkles around the mouth. It is composed of a gel that is smaller and smoother than other Restylane products, resulting in a softer, more precise result — ideal for restoring and enhancing the lips. Additionally, Silk is administered via ultrafine needle, which is designed for improved accuracy and precision.
Restylane Lyft is approved by the FDA to correct volume deficit in the back of the hands, for cheek augmentation and the correction of age-related midface contour deficiencies, as well as the treatment of moderate to severe facial folds, such as the nasolabial folds (smile lines). Lyft is the first and only hyaluronic filler approved by the FDA to treat face and hands.2,3 Lyft contains 0.3% lidocaine, which is added to reduce the discomfort during treatment.
Restylane is used to add volume and fullness to the skin to correct moderate to severe facial wrinkles and folds, such as the lines from your nose to the corners of your mouth (nasolabial folds). Restylane is also indicated for lip augmentation.
Restylane Refyne integrates with your skin to help smooth out the lines that run alongside the corners of your mouth (nasolabial folds and marionette lines)4 and the flexibility you need for natural-looking movement and expressions.5,6
Restylane Defyne is designed to act like your body’s own hyaluronic acid to help smooth out deeper lines around your mouth (nasolabial folds and marionette lines)4 while letting you look more natural through a range of facial expressions.7
Restylane products are approved only for patients over the age of 21.
Depending on your volume needs, most Restylane treatment appointments last less than an hour and begin to work almost immediately by adding volume to smooth away wrinkles. A clear gel formulation of hyaluronic acid, Restylane products act like your body’s own hyaluronic acid (and eventually break down naturally). Each Restylane product is designed for a specific purpose.
The filler is injected with a very small needle directly into the area to be treated. You may experience some stinging or burning as the filler is inserted. A series of treatment sessions may be necessary to fill and smooth lines and wrinkles, or to raise a depressed scar to the level of surrounding skin.
After your treatment, you may experience side effects, such as swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site and impaired hand function. These are typically mild in severity and normally last less than 7 days in nasolabial folds and less than 14-18 days in lips. Patients should be limited to 6.0 mL per treatment of moderate to severe facial wrinkles and folds. For treatment of volume deficit in the hands, patients should be limited to 3.0 mL per hand per treatment. Swelling may be more likely in patients under 36 years, and bruising may be more likely in patients over 35 years. Please see Important Safety Considerations for the Restylane family of products.
The effects of fillers can last anywhere between three months to two years. It depends on the product being used and where on the body the product is being injected as well as how the body metabolizes the filler.
With full treatment, you can see lasting results for up to 18 months in nasolabial folds with Restylane;10 up to 12 months in the cheeks, up to 6 months in the back of the hands, and up to 6 months in facial wrinkles and folds, such as nasolabial folds, with Restylane Lyft;3 6 months for lips and perioral rhytids with Restylane Silk;11 and up to 12 months in the facial wrinkles and folds (such as nasolabial folds and marionette lines) with Restylane Refyne and Restylane Defyne.
Restylane Silk: In clinical studies, the results of Restylane Silk lasted approximately 6 months following treatment. In a U.S. clinical trial involving 221 mostly female subjects, 98% of subjects reported a visible improvement in lip fullness in upper and lower lip (combined) 14 days after injection, and 77% still had lip improvement at 6 months following injection.11
Restylane Lyft: In a clinical trial involving 150 subjects, blinded evaluators observed that 90% of subjects treated with Restylane Lyft showed an improvement in fullness in the right and left midface areas (combined) at 2 months, and 54% of subjects showed an improvement at 12 months. Additionally, 95% of subjects reported that they were satisfied with the appearance of their midface at 2 months, and 73% of subjects were satisfied at 12 months.3 In a clinical trial involving 89 treated subjects, blinded evaluators observed that 86% of subjects* treated with Restylane Lyft showed an improvement in volume of the back of the hands at 3 months, and 76% of subjects* showed an improvement at 6 months. Improvement was defined as subjects with a Merz Hand Grading Scale (MHGS) score of ≥1 grade from baseline by the blinded evaluator’s live assessment.3
Restylane: In a clinical study treating the lines from the nose to the corners of the mouth (laugh lines), blinded evaluators reported that 95% of subjects improved for up to 18 months when they received an initial Restylane treatment, plus a follow-up treatment approximately 4.5 or 9 months later. Using this Restylane regimen of treatments, blinded evaluators reported similar subject improvement, on average, at 18 months compared to their Week 2 Wrinkle Severity Rating Scale (WSRS) score.10
Restylane Refyne & Restylane Defyne: In a clinical study of subjects treated for laugh lines with Restylane Refyne and Restylane Defyne, investigators reported improvement in the appearance of wrinkles for up to 12 months in the majority of patients. Using the Wrinkle Severity Rating Scale (WSRS), a validated 5-point measure of the size and depth of the wrinkles, with grade 1 defined as absence of wrinkles and grade 5 as extremely deep and long wrinkles, investigators reported that 79% of Restylane Refyne subjects and 77% of Restylane Defyne subjects had at least a 1-grade improvement on the WSRS after 6 weeks. Subjects also performed self-assessments (SSA) of wrinkle severity, with most reporting at least a 1-grade improvement in SSA scores with Restylane Refyne and with Restylane Defyne after 6 weeks.
Versa®
Revanesse Versa is hyaluronic acid dermal filler. Hyaluronic acid is a naturally occurring substance that is found within the body. It may be produced by bacteria and purified for use as injectable soft tissue filler in order to correct the appearance of facial wrinkles and creases, (nasolabial folds). The product is approved for use in the U.S. by the Food and Drug Administration for the cosmetic treatment of facial wrinkle and creases
The filler is injected with a very small needle directly into the area to be treated. You may experience some stinging or burning as the filler is inserted. A series of treatment sessions may be necessary to fill and smooth lines and wrinkles, or to raise a depressed scar to the level of surrounding skin.
Ask your doctor if you have questions about any of the side effects, and please tell your doctor or your doctor’s staff right away if you have any side effects. Please tell them if you have any problems with your health or the way you feel, whether or not you think these problems are related to the products
The most common side effects include: bruising, redness, swelling, pain and itching.
You should seek immediate medical attention if you develop symptoms such as unusual pain, vision changes, a white appearance of the skin near the injection site (blanching) or any other unexpected symptoms. While rare, unexpected symptoms include unusual pain, vision changes, or any signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms or legs, difficulty walking, visual changes, face drooping, severe headache, dizziness, or confusion) during or shortly after the procedure.
Rare, but serious risks, of dermal fillers include: scarring, blurred vision, partial vision loss, and blindness if the dermal filler is inadvertently injected into a blood vessel. In occasionally rare cases, there have been reports of unintentional injection of the product into a blood vessel with dermal filler products. It is recommended that doctors take care to avoid injection into blood vessels (especially around the forehead, nose and eye area) for those reasons, allergic reaction that may lead to a severe reaction (anaphylactic shock) that requires emergency medical help.
A Revanesse Versa treatment is minimally-invasive and provides immediate results. Take control of the signs of aging and ask your healthcare provider if Revanesse Versa is right for you.
You may find that Revanesse Versa will help in the improvement of your nasolabial folds (laugh lines) for up to 12 months with optimal correction.
With full treatment, you can see lasting results for up to 18 months in nasolabial folds with Restylane;10 up to 12 months in the cheeks, up to 6 months in the back of the hands, and up to 6 months in facial wrinkles and folds, such as nasolabial folds, with Restylane Lyft;3 6 months for lips and perioral rhytids with Restylane Silk;11 and up to 12 months in the facial wrinkles and folds (such as nasolabial folds and marionette lines) with Restylane Refyne and Restylane Defyne.
Restylane Silk: In clinical studies, the results of Restylane Silk lasted approximately 6 months following treatment. In a U.S. clinical trial involving 221 mostly female subjects, 98% of subjects reported a visible improvement in lip fullness in upper and lower lip (combined) 14 days after injection, and 77% still had lip improvement at 6 months following injection.11
Restylane Lyft: In a clinical trial involving 150 subjects, blinded evaluators observed that 90% of subjects treated with Restylane Lyft showed an improvement in fullness in the right and left midface areas (combined) at 2 months, and 54% of subjects showed an improvement at 12 months. Additionally, 95% of subjects reported that they were satisfied with the appearance of their midface at 2 months, and 73% of subjects were satisfied at 12 months.3 In a clinical trial involving 89 treated subjects, blinded evaluators observed that 86% of subjects* treated with Restylane Lyft showed an improvement in volume of the back of the hands at 3 months, and 76% of subjects* showed an improvement at 6 months. Improvement was defined as subjects with a Merz Hand Grading Scale (MHGS) score of ≥1 grade from baseline by the blinded evaluator’s live assessment.3
Restylane: In a clinical study treating the lines from the nose to the corners of the mouth (laugh lines), blinded evaluators reported that 95% of subjects improved for up to 18 months when they received an initial Restylane treatment, plus a follow-up treatment approximately 4.5 or 9 months later. Using this Restylane regimen of treatments, blinded evaluators reported similar subject improvement, on average, at 18 months compared to their Week 2 Wrinkle Severity Rating Scale (WSRS) score.10
Restylane Refyne & Restylane Defyne: In a clinical study of subjects treated for laugh lines with Restylane Refyne and Restylane Defyne, investigators reported improvement in the appearance of wrinkles for up to 12 months in the majority of patients. Using the Wrinkle Severity Rating Scale (WSRS), a validated 5-point measure of the size and depth of the wrinkles, with grade 1 defined as absence of wrinkles and grade 5 as extremely deep and long wrinkles, investigators reported that 79% of Restylane Refyne subjects and 77% of Restylane Defyne subjects had at least a 1-grade improvement on the WSRS after 6 weeks. Subjects also performed self-assessments (SSA) of wrinkle severity, with most reporting at least a 1-grade improvement in SSA scores with Restylane Refyne and with Restylane Defyne after 6 weeks.
Botox®
BOTOX® Cosmetic is the #1 selling treatment of its kind*:
- It’s the first and only treatment FDA-approved to temporarily make moderate to severe frown lines, crow’s feet and forehead lines look better in adults
- A quick 10-minute treatment with minimal downtime
- You may begin to notice results within 24 to 48 hours for moderate to severe frown lines
- It delivers predictable, subtle results, so you look like you, only with less noticeable facial lines
BOTOX® Cosmetic targets one of the underlying causes of frown lines, crow’s feet and forehead lines — the repeated muscle contractions from frowning, squinting, smiling and raising the eyebrows over the years. Your specialist will inject these muscles with BOTOX® Cosmetic to temporarily reduce muscle activity. You will begin to notice a visible smoothing of the frown lines between your brows, your crow’s feet lines and your forehead lines.
Three percent of patients experienced eyelid drooping in the frown lines studies, one percent of patients experienced eyelid swelling in the crow’s feet studies, and one percent of patients experienced brow drooping in the forehead lines studies. Other possible side effects include: dry mouth; discomfort or pain at the injection site; tiredness; headache; neck pain; eye problems: double vision, blurred vision, decreased eyesight and dry eyes; and allergic reactions. These are not all of the possible serious side effects of BOTOX® Cosmetic. Please see the Important Safety Information including Boxed Warning and Medication Guide and talk to your specialist.
You may begin to notice results within 24 to 48 hours, with results lasting up to four months for moderate to severe frown lines. Remember that results vary from patient to patient though, so your physician will plan your next appointment based on your results and aesthetic goals.
Start talking about your goals with a specialist today and find out what you could expect.
Dysport®
Dysport is a local muscle relaxant (or neuromuscular blocking agent) adapted from the botulinum toxin type-A and is used in both aesthetic and therapeutic indications.
Dysport is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adult patients less than 65 years of age.
Dysport is a prescription injection administered by a licensed healthcare professional to treat moderate to severe frown lines (those wrinkles between the eyebrows).
Although office visits can vary, the procedure generally takes 10 to 20 minutes.
Administered by a licensed healthcare professional, Dysport is supplied as a single-use, sterile 300-unit vial. The dose of Dysport for the treatment of glabellar lines (frown lines between your eyebrows) is a total of 50 units injected equally among the specific muscles. The dose of Dysport is not the same as the dose of any other botulinum toxin product and cannot be compared to the dosage of any other botulinum toxin product that you may have previously used.
The most common side effects are nose and throat irritation, headache, injection site pain, injection site skin reaction, upper respiratory tract infection, eyelid swelling, eyelid drooping, sinus inflammation, and nausea. For a full list of potential side effects, click here.
Though results vary, over half of users see improvement in moderate to severe frown lines between the brows within 2-3 days
One treatment of Dysport provides results that may last for up to 5 months
Kybella®
KYBELLA® is a prescription medicine used in adults to improve the appearance and profile of moderate to severe fat below the chin (submental fat), also called “double chin”.
It is not known if KYBELLA® is safe and effective for the treatment of fat outside of the submental area or in children under 18 years of age.
The active ingredient in KYBELLA® is synthetic deoxycholic acid. Deoxycholic acid is a naturally occurring molecule in the body that aids in the breakdown and absorption of dietary fat. When injected into the fat beneath the chin, KYBELLA® destroys fat cells, and once destroyed, these cells can no longer store or accumulate fat. This results in a noticeable reduction in fullness under the chin, revealing an improved chin profile.
The most common side effect of KYBELLA® include:
- Swelling, pain, numbness, redness, and areas of hardness in the treatment area.
- These are not
KYBELLA® can cause serious side effects including:
- Nerve injury in the jaw (which can cause an uneven smile or facial muscle weakness)
- Trouble swallowing
- Injection site problems including: bruising, hair loss, open sores (ulcers), damage and tissue cell-death (necrosis) around the injection site. Call your healthcare provider if you develop open sores or drainage from the treatment area
Please see KYBELLA® full Prescribing Information.
Please see accompanying full Prescribing Information, or ask your healthcare provider, or visit MyKybella.com
Your specialist will create a tailored treatment plan based on your chin profile. At each treatment, you will be given multiple small injections under your chin. You may receive up to 6 treatment sessions, spaced at least 1 month apart. Many patients experience visible results in 2 to
When injected into fat under the chin, KYBELLA® causes the destruction of fat cells. Once destroyed, those cells cannon store or accumulate fat. After reaching your desired aesthetic, further treatment is not expected.
Disclaimers
Juverderm—https://www.juvederm.com/
- The most commonly reported side effects with JUVÉDERM® injectable gels included redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching.
- For JUVÉDERM® VOLBELLA™ XC, dryness was also reported.
- For JUVÉDERM® VOLUMA™ XC, most side effects resolved within 2 to 4 weeks.
- For JUVÉDERM® VOLLURE™ XC, JUVÉDERM® XC, and JUVÉDERM® Ultra XC injectable gels, most resolved within 14 days or less.
- For JUVÉDERM® VOLBELLA™ XC, most resolved within 30 days or less. These side effects are consistent with other facial injection procedures.
- Most side effects will resolve with time. Your doctor may choose to treat side effects persisting over 30 days with antibiotics, steroids, or hyaluronidase (an enzyme that breaks down hyaluronic acid).
- One of the risks with these products is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin.
- As with all skin injection procedures, there is a risk of infection.
- Minimize strenuous exercise and exposure to extensive sun or heat within the first 24 hours following treatment. Exposure to any of these may cause temporary redness, swelling, and/or itching at the injection site
- Tell your doctor if you are pregnant or breastfeeding. The safety of these products for use during pregnancy or while breastfeeding has not been studied
- The safety of JUVÉDERM® VOLUMA™ XC has not been studied in patients under 35 years or over 65 years for cheek augmentation, or under 22 years and over 80 years for chin augmentation. The safety of JUVÉDERM® VOLLURE™ XC and JUVÉDERM® VOLBELLA™ XC has not been studied in patients under 22 years, and the safety of JUVÉDERM® XC and JUVÉDERM® Ultra XC has not been studied in patients under 18 years
The safety and effectiveness of JUVÉDERM VOLUMA® XC in areas other than the cheek area, JUVÉDERM® XC and JUVÉDERM VOLLURE™ XC for areas other than facial wrinkles and folds, and JUVÉDERM® Ultra XC and JUVÉDERM VOLBELLA® XC in areas other than the lips and perioral area have not been established in clinical studies - JUVÉDERM® VOLUMA™ XC is intended for use in the chin and cheek areas. JUVÉDERM® VOLLURE™ XC and JUVÉDERM® XC are intended for use in facial wrinkles and folds. JUVÉDERM® VOLBELLA™ XC and JUVÉDERM® Ultra XC are intended for use in the lips and perioral area. The safety and effectiveness for treatment in other areas have not been established in clinical studies
- Tell your doctor if you have a history of excessive scarring (thick, hard scars) or pigmentation disorders. The safety of JUVÉDERM® products has not been studied in these patients and may result in additional scars or changes in pigmentation
- Tell your doctor if you are on therapy used to decrease the body’s immune response (immunosuppressive therapy). Use may result in an increased risk of infection
- Tell your doctor before treatment if you are using substances that can prolong bleeding, such as aspirin, ibuprofen, or other blood thinners. As with any injection, this may result in increased bruising or bleeding at the injection site
- Patients who experience skin injury near the site of injection may be at a higher risk for adverse events
- JUVÉDERM® VOLUMA™ XC was not studied in patients with significant loose skin of the chin, neck, or jaw
- The effect of JUVÉDERM® VOLUMA™ XC injection into the chin on facial hair growth has not been studied
For more information, please visit:
Restylane— https://www.restylaneusa.com/resources/faqs#isi
The Restylane family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Refyne, Restylane® Defyne and Restylane® Kysse.
APPROVED USES
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane® Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane® Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds.
Do not use if you have severe allergies with a history of severe reactions (anaphylaxis), are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you have a history of scarring or pigmentation disorders as these side effects can occur with hyaluronic acid fillers. Tell your doctor if you are planning other cosmetic treatments (i.e., lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin, as these medications may increase the risk of bruising or bleeding at the gel injection site. Using these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete.
The most common side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
Versa— https://revanesseusa.com/
If you have an adverse inflammatory reaction, such as redness, pain and swelling that persist for one week or more after treatment with Revanesse Versa, you should report this immediately to your doctor. If you are under the age of 22 you should not be treated with Revanesse Versa.
One of the risks of using this product is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious, and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke temporary scabs, or permanent scarring of the skin. If you have changes in your vision, signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms, or legs, difficulty walking, face drooping, severe headache, dizziness, or confusion), white appearance of the skin or unusual pain during or shortly after treatment, you should notify your health care practitioner immediately.
Botox: https://www.botoxcosmetic.com
- Potency Units of BOTOX Cosmetic are not interchangeable with other preparations of botulinum toxin products
- Spread of toxin effects; swallowing and breathing difficulties can lead to death. Seek immediate medical attention if respiratory, speech or swallowing difficulties occur
- Potential serious adverse reactions after administration of BOTOX for unapproved uses
- Adverse event reports have been received involving the cardiovascular system, some with fatal outcomes. Use caution when administering to patients with pre-existing cardiovascular disease
- Use with caution in patients with compromised respiratory function or dysphagia
- The most common adverse reactions are:
- Glabellar Lines: eyelid ptosis (3%)
- Lateral Canthal Lines: eyelid edema (1%)
- Forehead Lines: headache (9%)
- Brow ptosis (2%)
Dysport: https://www.dysportusa.com/get-started/faq
Important Safety Information
What is the most important information you should know about Dysport? Spread of Toxin Effects: In some cases, the effects of Dysport and all botulinum toxin products may affect areas of the body away from the injection site. Symptoms can happen hours to weeks after injection and may include swallowing and breathing problems, loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, or loss of bladder control. Swallowing and breathing problems can be life threatening and there have been reports of death. You are at the highest risk if these problems are pre‐existing before injection.
These effects could make it unsafe for you to drive a car, operate machinery, or do other dangerous activities.
Do not have Dysport treatment if you: are allergic to Dysport or any of its ingredients (see the end of the Medication Guide for a list of ingredients), are allergic to cow’s milk protein, had an allergic reaction to any other botulinum toxin product, such as Myobloc®, Botox®, or Xeomin®, have a skin infection at the planned injection site, under 18 years of age, or are pregnant or breastfeeding.
Kybella
Marginal mandibular nerve (MMN) injury: Follow injection technique to avoid this injury.
Dysphagia may occur with KYBELLA use. Use in patients with preexisting dysphagia may exacerbate the condition.
Submental hematoma/bruising occurs frequently after KYBELLA administration. Use with caution in patients who are being treated with antiplatelet or anticoagulant therapy or have coagulation abnormalities.
Avoid injecting in proximity to vulnerable anatomic structures due to the increased risk of tissue damage and vascular injury.
Injection site alopecia: Withhold subsequent treatments until resolution.
Injection site ulceration and necrosis: Do not administer to the affected area until complete resolution.